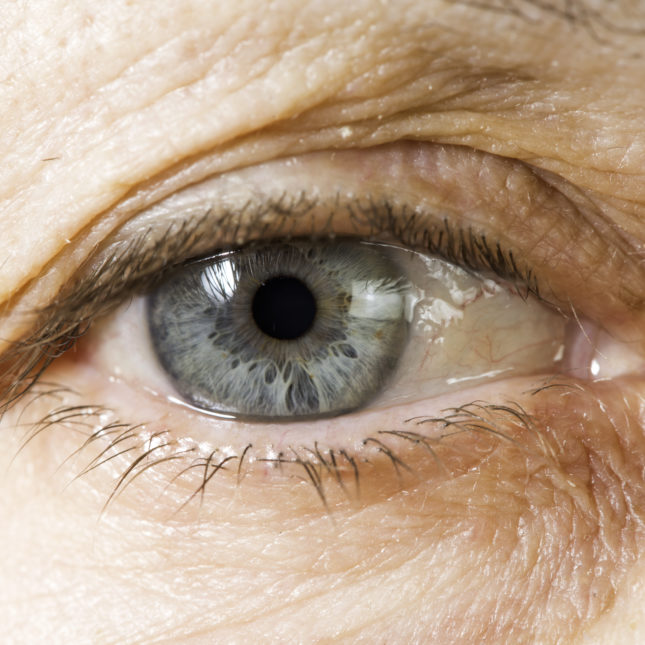
Due to a shortage of a widely used compounded medicine for serious eye diseases, two leading ophthalmologic groups want Medicare contractors to halt practices that limit coverage of other — albeit costlier — treatments over concerns about patient access.
At issue is Avastin, an older cancer medicine that is often used by physicians to treat age-related macular degeneration and macular edema, among other ailments. The injectable medication was never approved for these purposes, but has proven effective, and so vials are often repackaged. Moreover, it is much cheaper than treatments that were approved by regulators for those uses.
Earlier this month, though, a company that is the largest supplier of repackaged Avastin began recalling its supplies after the U.S. Food and Drug Administration inspected its facilities and found sterility issues. In Oct. 2 letters to customers, Pine Pharmaceuticals, which also compounds medicines, did not offer a timeline for when it could resume repackaging and shipping Avastin.













Exciting news! STAT has moved its comment section to our subscriber-only app, STAT+ Connect. Subscribe to STAT+ today to join the conversation or join us on Twitter, Facebook, LinkedIn, and Threads. Let's stay connected!
To submit a correction request, please visit our Contact Us page.