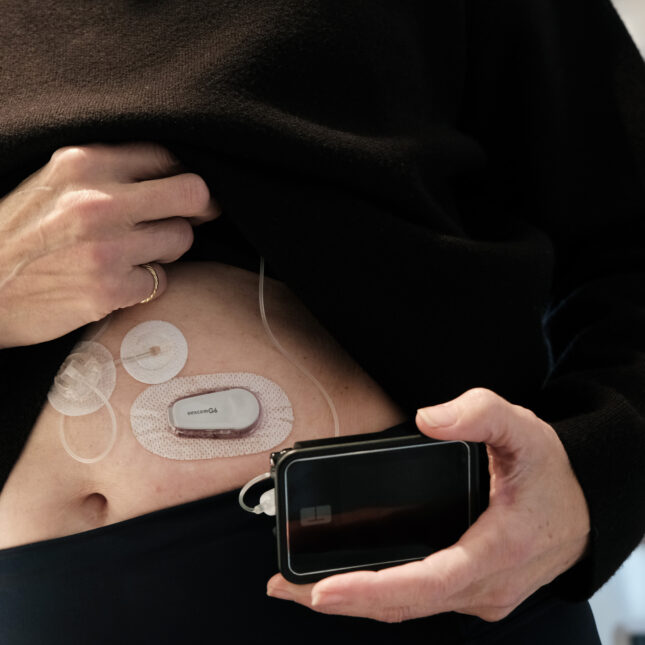
When Dana Lewis built her first artificial pancreas in 2014, she — and millions of other people with type 1 diabetes — didn’t have another option. Back then, diabetes device manufacturers hadn’t closed the loop between the blood glucose sensors that help determine a person’s next insulin dose and the pumps that deliver it. So she took the reins, hacking her existing devices to build a DIY system that could automate her insulin doses.
Since then, the community of DIYers with type 1 diabetes has grown to the thousands. But today, they have alternatives: Device manufacturers have caught up with their own, FDA-cleared closed-loop systems, and automated insulin delivery is becoming more common. In a world with these commercial options, does the DIY artificial pancreas still have a place?
The answer, said pediatric endocrinologist Greg Forlenza in a debate with Lewis on Friday at this year’s American Diabetes Association scientific sessions, is no. With four approved systems on the market, he argued, there’s no need to take on the risk of linking together a hodgepodge of tools with an algorithm that itself is unapproved by the FDA.














Exciting news! STAT has moved its comment section to our subscriber-only app, STAT+ Connect. Subscribe to STAT+ today to join the conversation or join us on Twitter, Facebook, LinkedIn, and Threads. Let's stay connected!
To submit a correction request, please visit our Contact Us page.