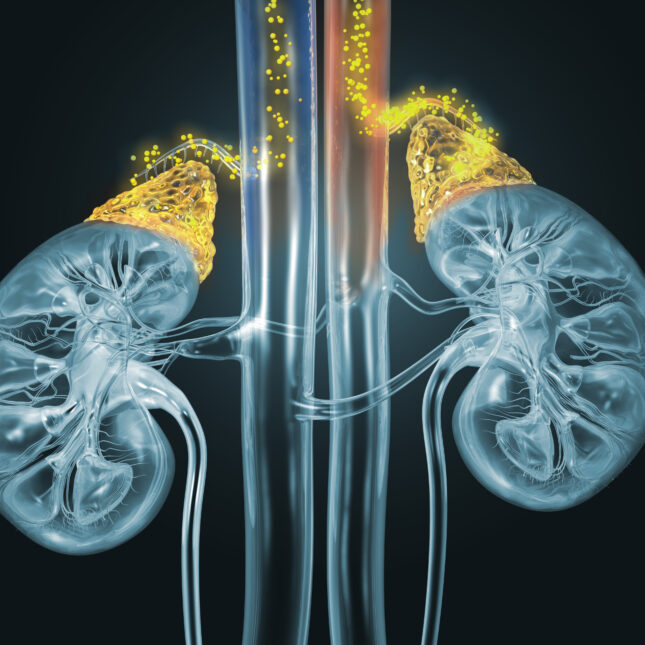
SAN DIEGO — Neurocrine Biosciences, a biotech focused on treating rare neurological and endocrine diseases, announced on Thursday that its experimental drug for congenital adrenal hyperplasia succeeded in a late-stage trial of children and adolescents, marking the second success the company has reported in the past month for a drug observers believe has blockbuster potential.
The Phase 3 study, dubbed CAHtalyst Pediatric, recruited 103 children and adolescents with the most common form of the disease. Patients were randomly assigned to receive either a placebo or crinecerfont, an oral drug designed to lower male sex hormones produced in excess by people with the disease. Those given the drug had significantly lower levels of the hormone androstenedione after four weeks, which was the study’s main endpoint. Researchers also found that 30% of people taking crinecerfont lowered their dose of the current standard-of-care medications, glucocorticoids, to levels researchers say would be safer for long-term use, while none on placebo did so.
The treatment was generally safe, and the most common adverse events were mild and included headache, fever, and vomiting.













Exciting news! STAT has moved its comment section to our subscriber-only app, STAT+ Connect. Subscribe to STAT+ today to join the conversation or join us on Twitter, Facebook, LinkedIn, and Threads. Let's stay connected!
To submit a correction request, please visit our Contact Us page.